At the Bench: The High Definition family of retinal ganglion cells
By Dr Jason Jacoby
Historically, the diverse group of ~40 retinal ganglion cells (RGCs) are divided into subtypes by either their distinct morphology or their ability to extract specific features from a visual scene. Subtypes that are labeled as “feature detectors” respond especially strongly or selectively to things such as local or global motion, direction of motion, stimulus orientation, contrast or uniformity, or the presence of large or small objects.
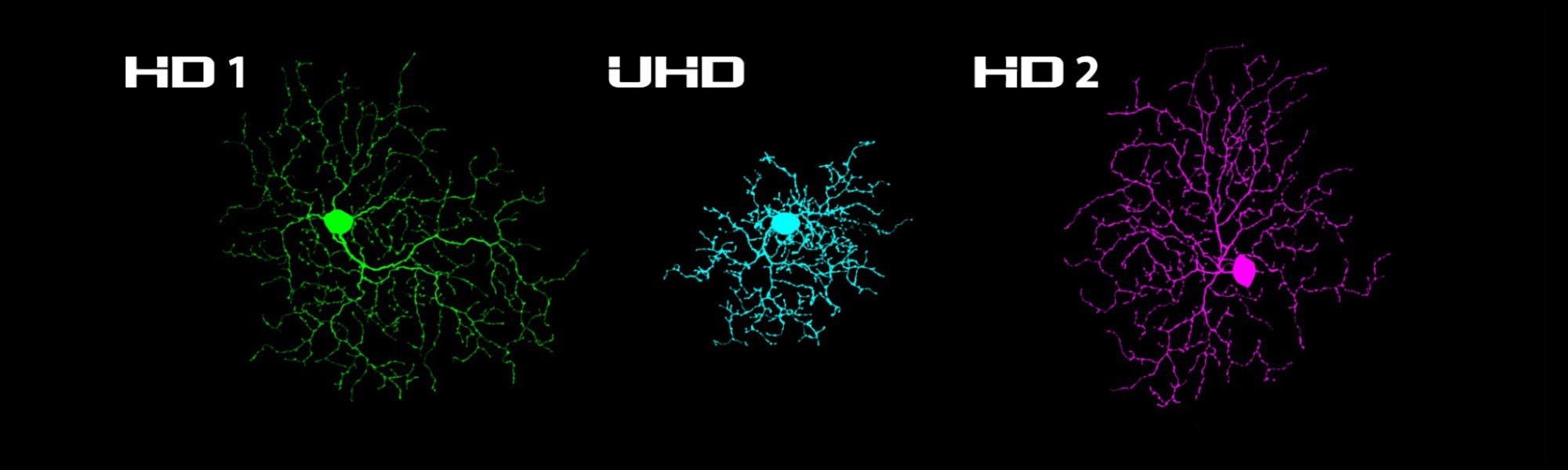
We recently identified three novel, small-receptive-field, non-direction selective ON-OFF retinal ganglion cells in the mouse retina through our research efforts at Northwestern University. Each type has a feature detection profile that is individually tuned to size, speed, and object motion. But perhaps most notably, these RGCs can detect small objects more readily than other cell types because they possess small dendritic arbors, small receptive field centers, and strong surround suppression.
The small receptive and dendritic fields of these three cell types imply that they may be among the most densely populated RGC types in the mouse retina; thus we named them “High Definition” RGCs based on the structure of pixel densities in modern displays.
The area upon which a visual stimulus falls upon the retina to maximally excite these cells is small, and if the object is larger than the optimally sized stimulus, strong surround suppression greatly reduces the response amplitude. When the visual stimulus surpasses a certain size, the cell becomes completely unresponsive. The most extreme case of this size-dependency in the manuscript can be taken from the Ultra High Definition (UHD) RGC; this cell type responds maximally to spots of light less than 90 mm in diameter, but if the spot exceeds 250 mm in diameter, the cell becomes silent.

Our paper, recently published in the Journal of Neuroscience, furthers the characterisation of the three HD RGC types (HD1, HD2, and UHD) by using electrophysiological approaches to identify their highly-stereotyped responses to light, their receptive field size and structure, and the pre- and post-synaptic mechanisms that contribute to their strong surround suppression. Furthermore, we provide strong evidence that these cells are distinct from each other and other RGCs described in the literature (namely the Local Edge detector (LED) RGC).
By using a custom designed light-projection device setup recently described in the SciMethods application note by Scientifica entitled, “Visual Stimulation of Retinal Explants on a Standard Multiphoton Microscope”, complex visual stimuli were used to probe each cell’s ability to respond to bars moving across their receptive fields at different speeds and sizes, their ability to discern textures of different spatial scales, and their ability to respond to global or differential motion (center and surround portions moving out of phase).
The experiments and computer modeling we performed in this research article show that while these three HD RGCs share certain receptive field properties, they also have distinct tuning to the size, speed, and type of motion on the retina, allowing them to occupy different niches in visual stimulus space.
The discovery of this family of High Definition RGCs lends progress to the goal of researchers around the world to formulate a complete ‘parts list’ of retinal neurons. Understanding how specific cell types are wired to each other in the retina should lay the foundation for targeted clinical therapies to combat retina-based vision loss.
Read the full manuscript via The Journal of Neuroscience website
About the author:
Dr Jason Jacoby began his career in vision in 2004 while surgically removing human corneas from deceased donors for transplant, ultimately leading to the restoration of sight to those blinded by preventable forms of corneal blindness. He went on to pursue his PhD in retinal neurobiology at the University of Illinois at Chicago where he studied lateral inhibition mediated by horizontal cells in the outer retina. In 2014, Dr Jacoby accepted a postdoctoral fellowship at Northwestern University where he obtained the Ruth L. Kirschstein National Research Service Award (NRSA F32) from the National Eye Institute to support future research efforts. He currently investigates the synaptic connectivity and signaling properties of retinal amacrine cells, as well as the identification and functional significance of novel retinal ganglion cell subtypes. Dr Jacoby’s primary research interests include multiple-electrode patch-clamp electrophysiology, functional fluorescent imaging, optogenetics, and hopes to one day have a hand in restoring sight to victims of retinal-blindness.

)